VenaSeal™ Procedure Nonthermal Vein Closure

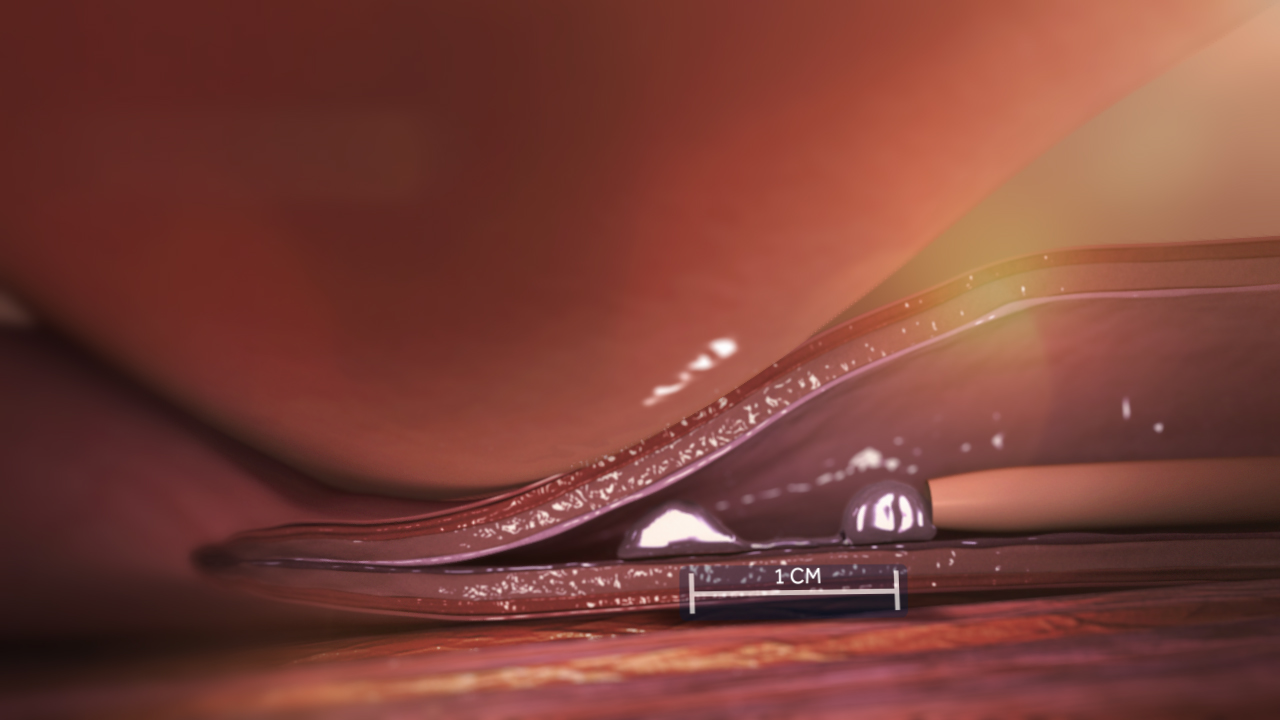

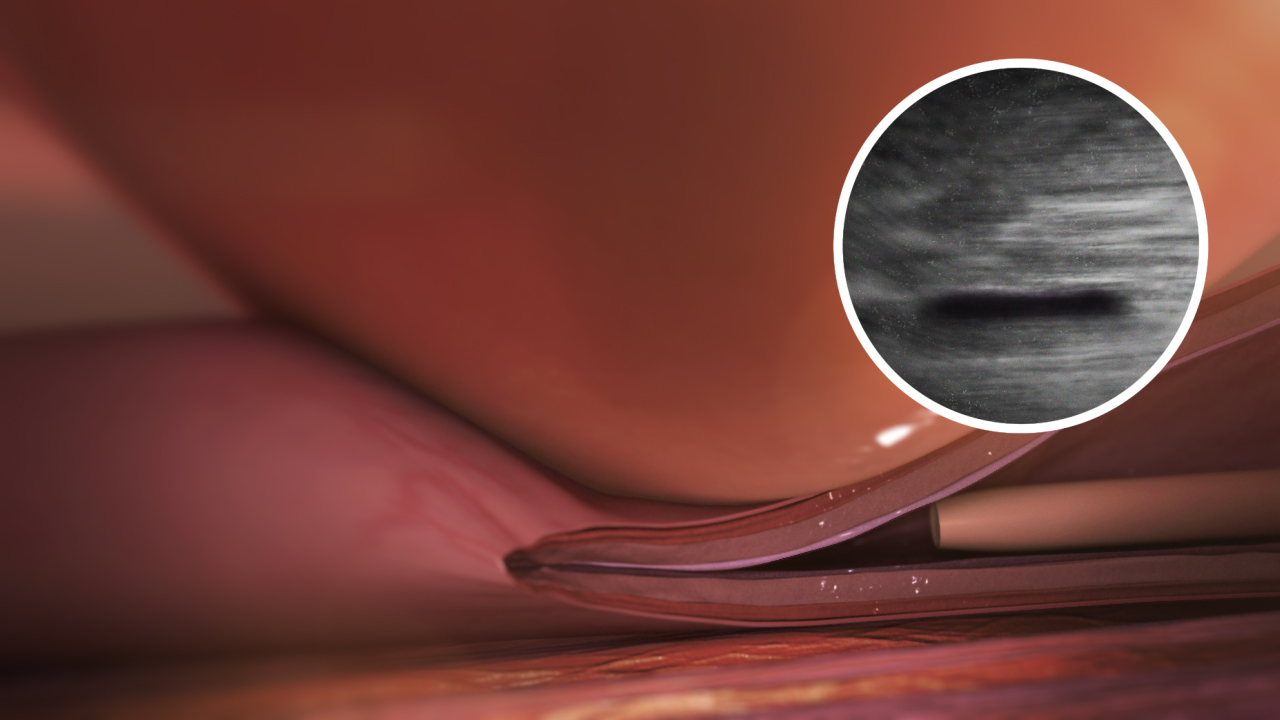
The VenaSeal™ Closure System is an innovative, minimally invasive treatment for varicose veins. It uses a proprietary medical adhesive to seal the affected veins, eliminating the need for heat, sclerosants, or tumescent anesthesia. This approach significantly reduces discomfort during and after the procedure. Unlike thermal ablation techniques, VenaSeal also eliminates the risk of nerve injury, particularly when treating the small saphenous vein. The procedure is performed in-office, requires minimal recovery time, and has been clinically proven to be safe, effective, and well-tolerated by patients.
Demonstrated Outcomes:
The VenaSeal closure system is a safe and effective treatment, providing significant improvement in quality of life. In a US. study, the VenaSeal system and thermal radiofrequency ablation treatments had similar clinical results at five years: 94.6% closure for the VenaSeal system® and 91.9% for thermal energy .
The VenaSeal System
It delivers a small amount of a specially formulated medical adhesive to close the diseased vein, rerouting blood to nearby healthy veins, which provides symptom relief.
Before:
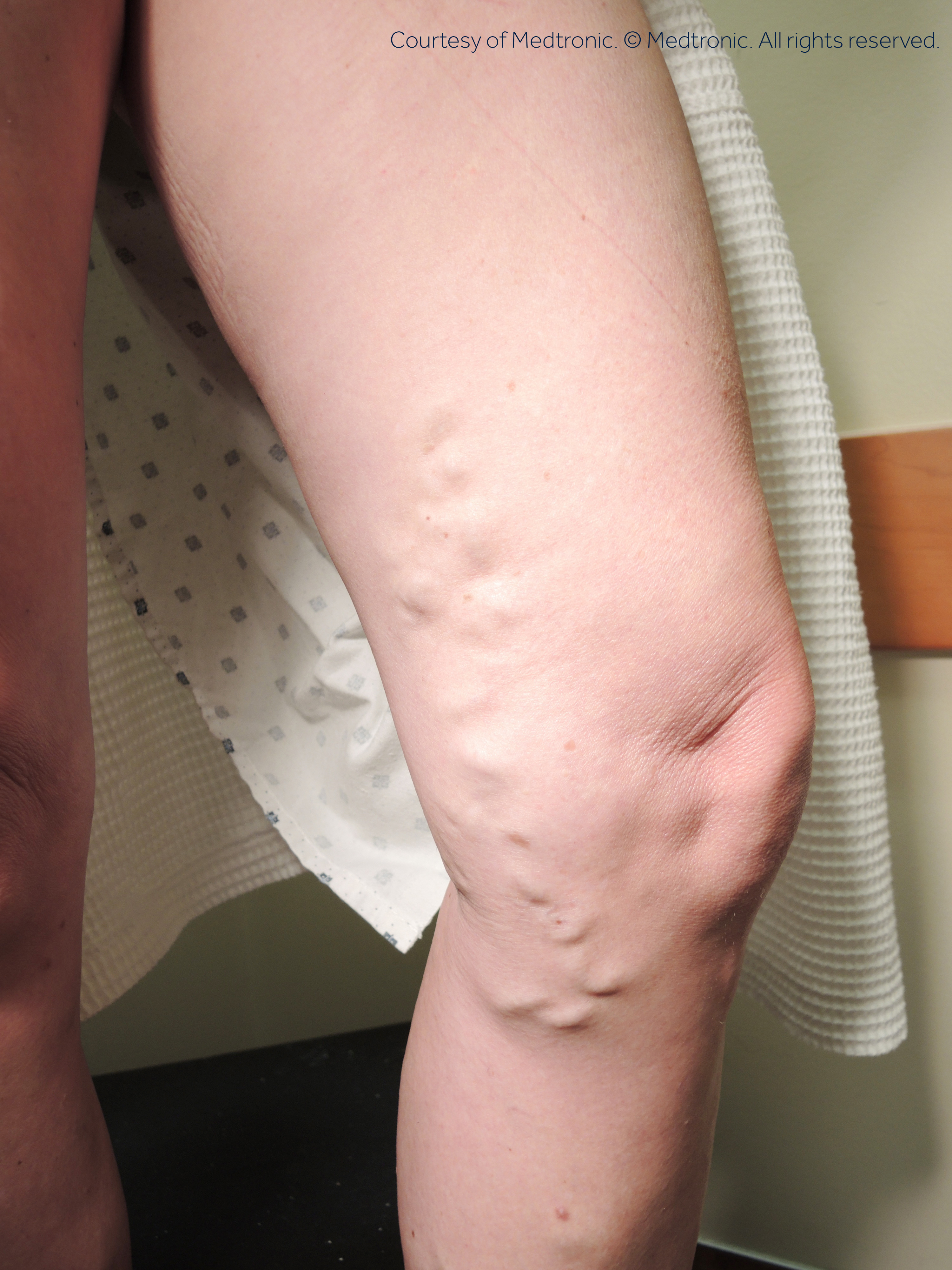
After:

Learn More About VenaSeal™ Procedure
A more comfortable experience
- Simple, outpatient procedure
- No tumescent anesthesia
- Less pain and bruising than thermal ablation.
- Faster recovery time than thermal ablation.
- Compression stockings not needed after the procedure
May not be for you :
The VenaSeal procedure is contraindicated for individuals with any of the following conditions:
- Thrombophlebitis migrans (i.e., inflammation of a vein caused by a slow-moving blood clot)
- Acute superficial thrombophlebitis (i.e., inflammation of a vein caused by a blood clot)
- Previous hypersensitivity reactions to the VenaSeal adhesive or cyanoacrylates
- Acute sepsis (i.e., whole-body inflammation caused by an immune response to an infection)
Potential risks
The VenaSeal procedure is minimally invasive and catheter-based. As such, it may involve the following risks. Your doctor can help you understand these risks.
- Adverse reactions to a foreign body (including, but not limited to, nonspecific mild inflammation of the cutaneous and subcutaneous tissue)
- Arteriovenous fistula (i.e., an abnormal connection between an artery and a vein)
- Bleeding from the access site
- Deep vein thrombosis (i.e., blood clot in the deep vein system)
- Edema (i.e., swelling) in the treated leg
- Embolization (i.e., blockage of a vein or artery), including pulmonary embolism (i.e., blockage of an artery in the lungs)
- Hematoma (i.e., the collection of blood outside of a vessel)
- Hyperpigmentation (i.e., darkening of the skin)
- Hypersensitivity or allergic reaction to cyanoacrylates, such as urticaria, shortness of breath, and anaphylactic shock
- Infection at the access site
- Pain
- Paresthesia (i.e., a feeling of tingling, pricking, numbness, or burning)
- Phlebitis (i.e., inflammation of a vein)
- Superficial thrombophlebitis (i.e., inflammation of a vein caused by a blood clot)
- Urticaria (i.e., hives), erythema (i.e., redness), or ulceration may occur at the injection site
- Vascular rupture and perforation
- Visible scarring
VenaSeal closure system kit
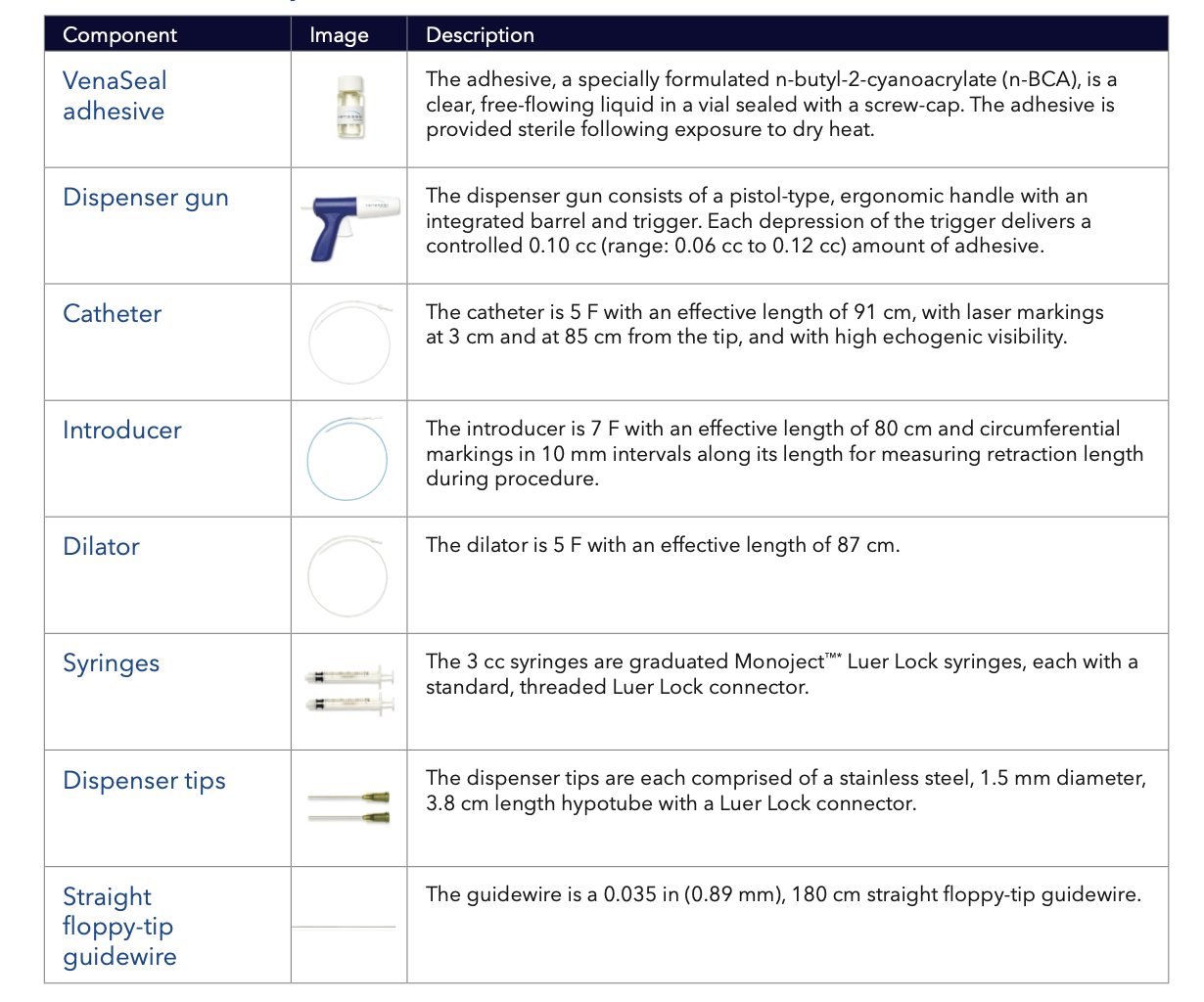
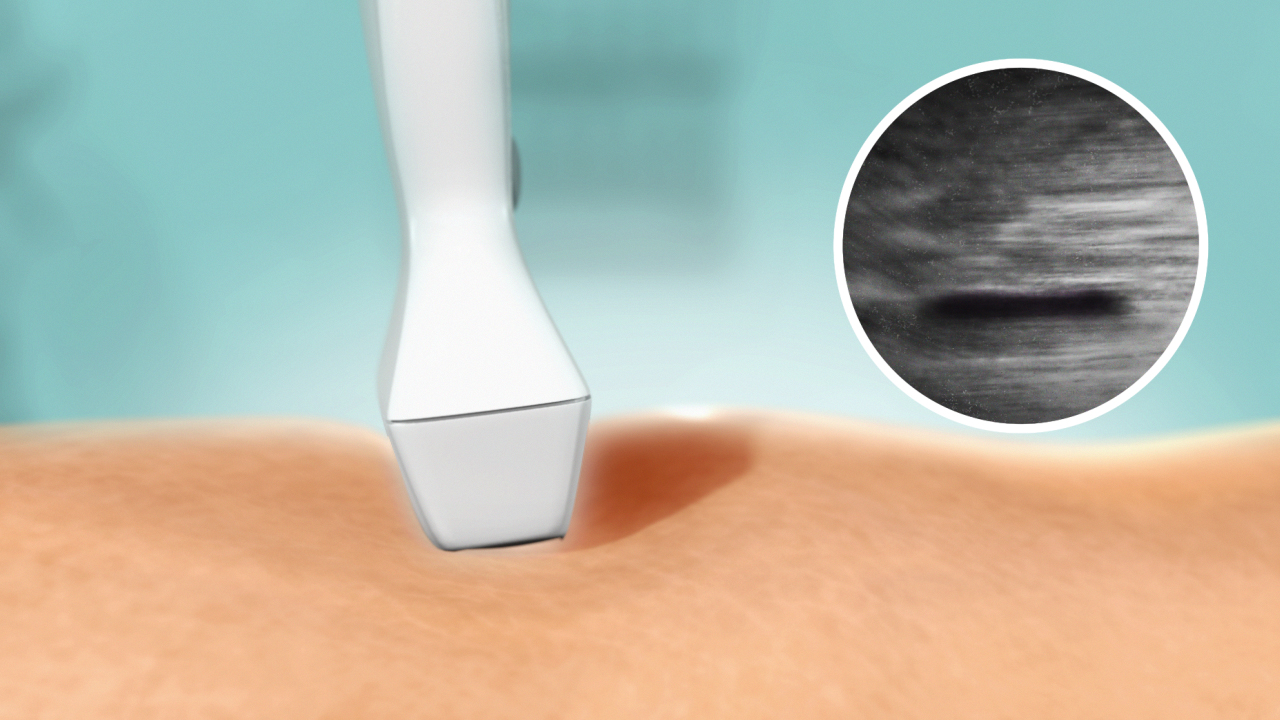
VenaSeal Procedure External Compression with Ultraound
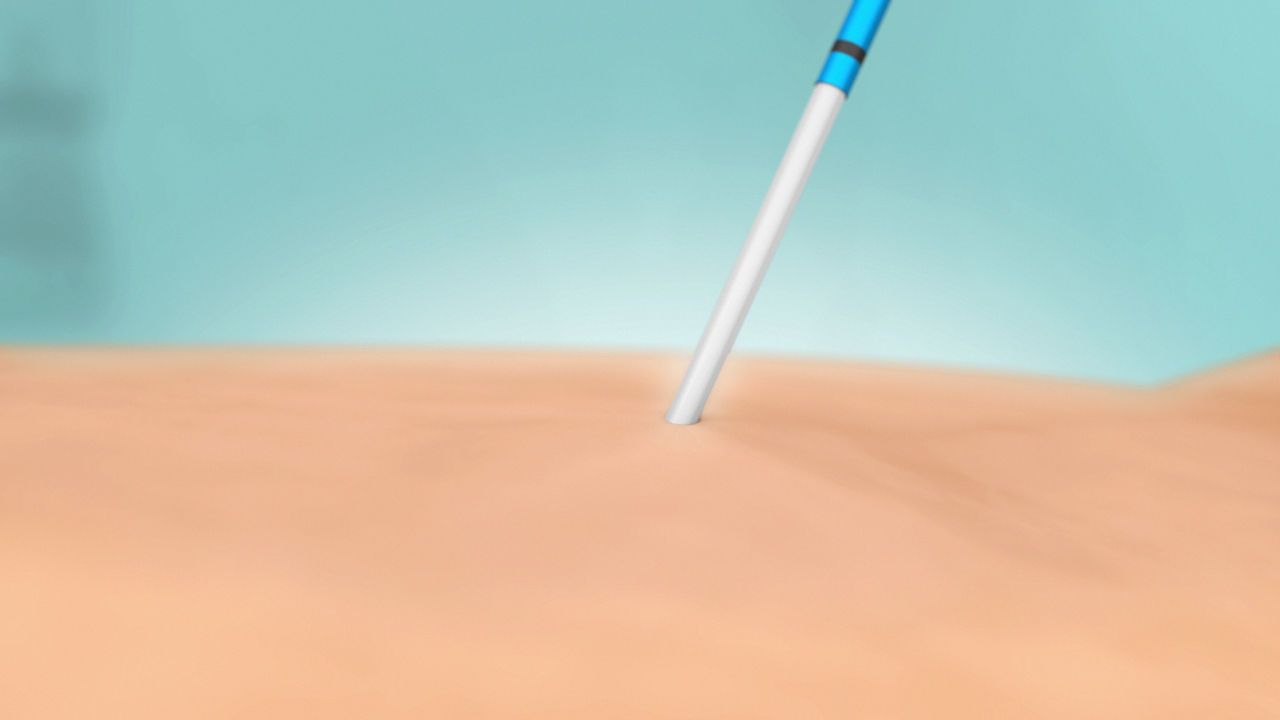
VenaSeal Procedure Catheter Removal
Brief statement VenaSeal™ closure system
Intended Use/Indications: The VenaSeal™ closure system (VenaSeal system) is indicated for use in the permanent closure of lower extremity superficial truncal veins, such as the great saphenous vein (GSV), through endovascular embolization with coaptation. The VenaSeal system is intended for use in adults with clinically symptomatic venous reflux as diagnosed by duplex ultrasound (DUS).
Contraindications: Separate use of the individual components of the VenaSeal closure system is contraindicated. These components must be used as a system. The use of the VenaSeal system is contraindicated when any of the following conditions exist: previous hypersensitivity reactions to the VenaSeal adhesive or cyanoacrylates, acute superficial thrombophlebitis, thrombophlebitis migrans, acute sepsis.
Potential Adverse Effects of the Device on Health: The potential adverse effects (e.g., complications) associated with the use of the VenaSeal system include, but are not limited to, adverse reactions to a foreign body (including, but not limited to, nonspecific mild inflammation of the cutaneous and subcutaneous tissue), arteriovenous fistula, bleeding from the access site, deep vein thrombosis (DVT), edema in the treated leg, embolization, including pulmonary embolism (PE), hematoma, hyperpigmentation, hypersensitivity or allergic reactions to cyanoacrylates, such as urticaria, shortness of breath, and anaphylactic shock, infection at the access site, pain, paresthesia, phlebitis, superficial thrombophlebitis, urticaria, erythema, or ulceration may occur at the injection site, vascular rupture and perforation, visible scarring.
Frequently Asked Questions
VenaSeal is a non-thermal, non-tumescent, minimally invasive procedure used to treat chronic venous insufficiency and varicose veins. It uses a medical adhesive to close abnormal veins, rerouting blood flow through healthy veins.
Return quickly to an active lifestyle!
